Indicator For Sodium Nitrate Titration
Ferroin indicator and acid is added to the sample. The difference between the solubilities of two silver salts.

Acids Bases Salts A Guide For Gcse Students
The Mohr titration method uses the standard solution of silver nitrate and the silver complexes with the chloride in our sample react with the chromate added to the sample to form silver chromate.
Indicator for sodium nitrate titration. Place 20 mL of hydrochloric acid 250 gl TS and 50 mL of water in the titration vessel add the quantity of the test substance and if indicated a catalyst as specified in the monograph and stir to dissolve. Another widely used indicator is xylenol orange. The indicator gives a clear colour.
The titration was carried out at a pH between 7 and 10 because chromate. The nitrate ion NO_3- is a very weak base that would be protonated only under strongly acidic conditions. A Versatile and General One-Pot Method for Synthesizing Bis-spiroketal Motifs.
The titration may be carried out manually or by means of an automatic titrator. Acid-base titration would not be suitable for NaNO_3. The assay with iodideThe standard solution of silver nitrate is placed in a burette divided into tenths of a cc.
Silver chloride is precipitated quantitatively before red silver chromate is formed. A solution of 10000mL of 02000molL sodium carbonate and 20000mL of 01000molL calcium nitrate solutions are mixed together according to the reaction. This is a conventional acid-base indicator into which iminodiacetic acid groups have been introduced thus permitting the substance to act as a metal-complexing indicator.
In a neutral or slightly alkaline solution potassium chromate can indicate the end-point of the silver nitrate titration of chloride. Weigh accurately 75 g of sodium nitrite and add sufficient DW to produce 1 litre in a 1000 ml volumetric flask. PREPARATION OF 01 M SODIUM NITRITE SOLUTION.
An Ingold saturated calomel electrode with a liquid junction tube filled with 050 M sodium nitrate was used as a reference electrode. 55 Potassium chromate indicator solution. The amount of silver reacted with chloride is used to determine how much sodium was in our sample.
Sodium carbonate calcium nitrate calcium carbonate sodium nitrate. You should multiply your titre by 065. Dissolve 50 g of potassium chromate K 2Cr0 4 in 100 mL of reagent water and add silver nitrate AgN0 3 until a slightly red precipitate is produced.
The quantity of titrant used changes in relation to the concentration of sodium nitrite in the sample. STANDARDIZATION OF 01 M SODIUM NITRITE SOLUTIOlN WITH. The Macrolide Toxin Mycolactone Promotes Bim-Dependent Apoptosis in Buruli Ulcer through Inhibition of mTOR.
The endpoint of the titration is identified as the first appearance of a red-brown colour of silver chromate figure 2. Thermoreversible Partitioning of Poly ethylene oxides between Water and a Hydrophobic Ionic Liquid. Also your multiplication factor looks like the one for sulphuric acid.
Cool to about 15 C and titrate slowly with sodium nitrite 01 moll VS placing the burette tip below the surface of the solution. Of standard silver nitrate. The sample is titrated with tetravalent cerium ion which is a strong oxidant.
In the manual titration add 01 M sodium nitrite slowly and when the titration is within 1 ml of the endpoint add the titrant in 01 ml portions allowing not less than 1 minute between additions. Make up a 5 or 01 mol dm-3 solution of the indicator and repeat the titration using the indicator. The prussic acid found would be equivalent to.
This works for a 10ml vat sample titrated with 10N sodium Hydroxide and give you a result expressed as percent by volume of 70 700gl0 nitric acid. 1 per cent of potassium cyanide. Silver nitrate in the presence of a few drops of potassium chromate solution as indicator is a simple direct and accurate method for chloride determination.
Sodium chloride is very slightly hygroscopic and for accurate work it is best to dry the solid at 140oC prior to use. Figure 1 Before the addition of any silver nitrate the chromate indicator. In this experiment the amount of chloride in an unknown sample was determined by Mohr titration.
A few typical examples are described below to get an indepth knowledge about sodium nitrite titrations. Suppose for example the difference in the two titrations equals 1 cc. The indicator electrodes were an Ingold glass electrode 401 a copper amalgam electrode and a copper-selective Selectrode Radiometer.
Add the indicator to the chloride solution. The layout of the titration system is shown in Fig. The indicator you should use is bromocresol green.
Allow the solution to stand protected from light for at least 24 hours after the addition of AgN0 3. Late stage in the titration. So the sudden appearance of the red silver chromate can be used to indicate the end point of the titration.
Silver chloride and silver chromate and their different colors is used to determine the end poi. Repeat the titration with further aliquots of diluted seawater until concordant results titres agreeing within 01 mL are obtained. The galvanometer needle deflects and then returns to approximately its original position until the end point is reached.
54 Phenolphthalein indicator solution 10 gL. After the cerium oxidizes the nitrite the indicator is oxidized and causes a color change from orange to pale blue. Therefore acid-base titration would be an unsuitable method to analyze NaNO_3 solutions.
A particular vinegar contains 40.
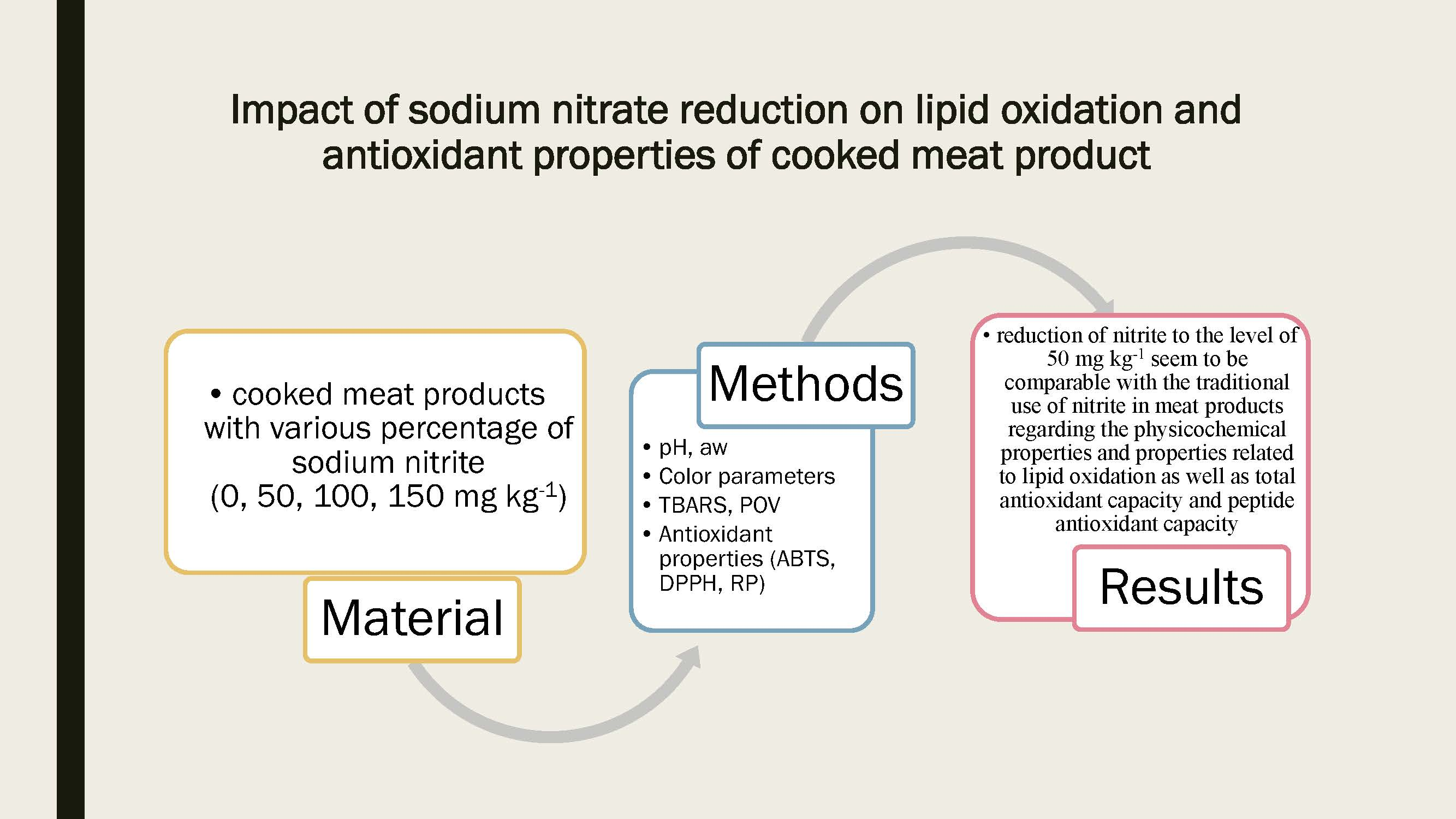
Antioxidants Free Full Text Impact Of Sodium Nitrite Reduction On Lipid Oxidation And Antioxidant Properties Of Cooked Meat Products Html

Chapter 5 Water And Solution Physical Characteristics Of

Acids Bases Salts A Guide For Gcse Students
Chemical Changes And Structure Neutralisation Learning Intentions We
Gcse Chemistry Year 10 Preparation Of Salts Page

Acids Bases Salts A Guide For Gcse Students

Is Nano3 Acidic Basic Or Neutral Dissolved In Water Youtube

Acids Bases And Salts Learning Outcomes Chapter Ppt Download

Acids Bases Salts A Guide For Gcse Students

Learning Objectives Describe And Explain The Tests For Ions Using Sodium Hydroxide Solution Explain How Precipitation Reactions Can Be Used To Test For Ppt Download

How To Make Sodium Nitrate A Potassium Nitrate Substitute Potassium Nitrate Survival Prepping Survival Techniques

Pdf Assay For Sodium Nitrite By Acidimetry And Its Comparison With Redox Titrimetric Assay
Catalysis Of A Sodium Thiosulfate And Iron Iii Nitrate Reaction Experiment Rsc Education

Is Nano3 Acidic Basic Or Neutral Dissolved In Water Youtube
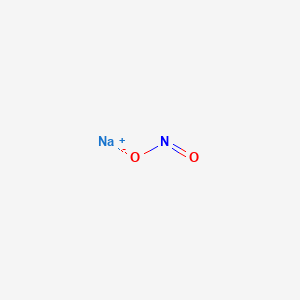
Sodium Nitrite 0 1m Standardized Solution Uses Dmf Dossier Manufacturer Supplier Licensing Distributer Prices News Gmp

Ks4 Chemistry Chemical Reactions Ppt Video Online Download

Post a Comment for "Indicator For Sodium Nitrate Titration"