How To Identify A Redox Equation
A g N O X 3 N a C l A g C l N a N O X 3 B. X 4-2 -1.
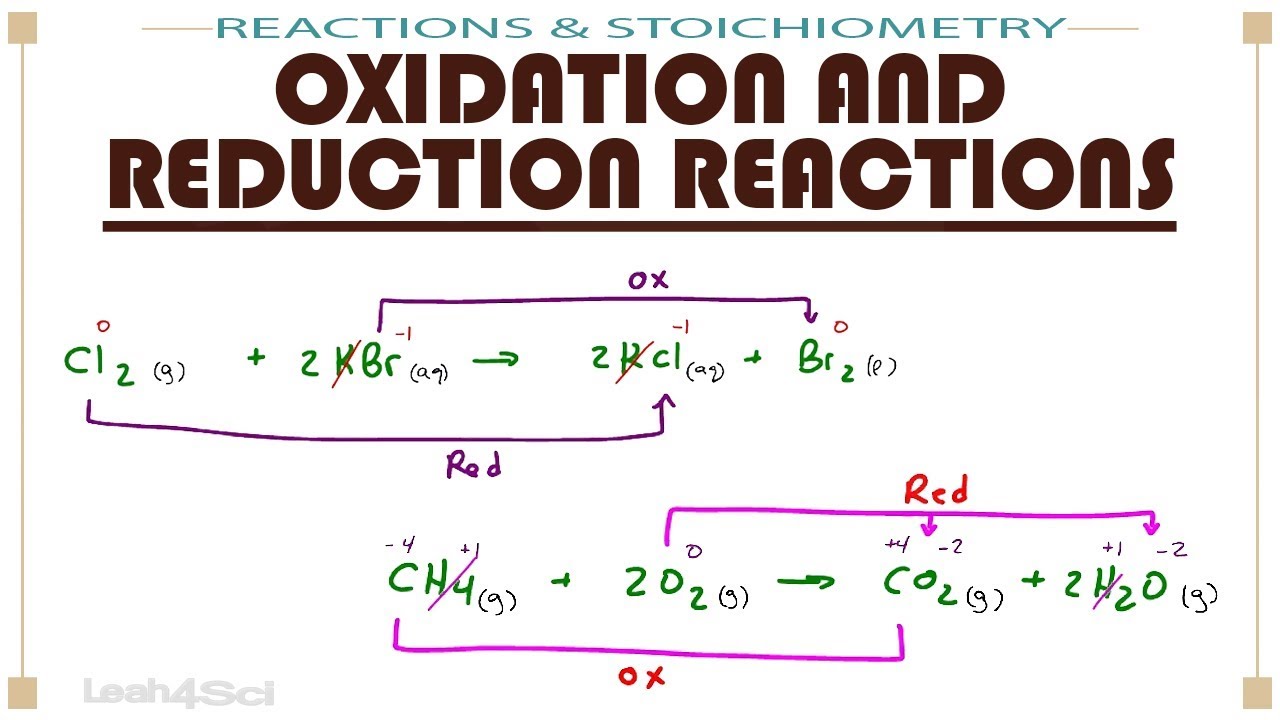
Oxidation And Reduction Redox Reactions Youtube
D shows an acid-base reaction.

How to identify a redox equation. Write the half reactions. H2 O22- - H2O. Assign oxidation numbers to each of the atoms.
I dont understand how to identify a redox reaction. Eqrm 2MnO_4-aq 3S2-aq 4H_2Ol to 3Ss 2MnO_2s 8OH-aq eq To identify the oxidized and reduced atoms we must. If any of the oxidation numbe.
Not redox ions K Cl--- KCl watch out for the IONS. Considering the equation above we have 2 hydrogen H with the total charge 1 Refer the charges of the elements in the above table and 2 oxygen O with the total charge -2 on the LHS and 2 hydrogen H with total charge 2 and only 1 oxygen O with the total charge -2 on the RHS. In this video you will figure out how to find oxidation numbers oxidizing agents reducing agents the substance being oxidized and the substance being redu.
This is done by assigning oxidation numbers to each atom before and after the reaction. If there is a change in oxidation number then the reaction is a redox reaction. The equation can be split into two parts and considered from the separate perspectives of the elemental magnesium and of the copper II ions.
C u O C O C u C O X 2 D. Oxidizing and reducing agents. Balancing a redox equation in basic solution.
Is a double displacement reaction a redox reaction. The oxidation state helps you to keep track of the movement of electrons in a redox process It is written as a - sign followed by a number. Posted on August 10 2019 To identify a redox reaction we must first calculate the oxidation number of each atom in the reaction.
Regents redox reactions have a Free element on one side of a reaction and the same element bonded to something on the other side. The given balanced overall redox reaction equation is. Eg AgNO3NaClAgClNaNO3 Ag is 1 before and after the reaction.
To determine the oxidation state of Mn in MnO 4- apply Equation 1 see Equation 1 above. M g M g 2 2 e. Balancing a redox equation in acidic solution.
C u 2 M g C u M g 2. Example- 2H 2 O 2-- 2H 2 O Free element --- Bonded element. It is not written as O 2- as this refers to the ion and its charge.
Electron Oxidation-reduction oxidize 2 more redox reduce. For example in NO 3 the nitrogen is assigned an oxidation number of 5 and each oxygen an oxidation number of 2. H C l K O H K C l H X 2 O.
Which species is oxidized and which species is reduced. SCICHE99906 Identifying Redox Reactions - Chemistry. B a C l X 2 K X 2 C O X 3 B a C O X 3 2 K C l C.
For it to be a redox reaction elements have to change oxidation states and that does not happen in double replacement reactions. This arrangement clearly indicates that the magnesium has lost two electrons and the copper II ion has gained them. The 4 is from the number of oxygen atoms -2 is the oxidation state of oxygen and -1 is the overall charge of the molecule.
If there is no change in oxidation number then the reaction is not a redox reaction. How can you determine if a given chemical reaction is a redox reaction or not. Oxidizing and reducing agents.
Example 2 2KClO 3-- 2KCl 3O 2 Bonded oxygen ---- Free oxygen. Our mission is to provide a free world-class education to anyone anywhere. Equations for redox reactions can be produced by adding together the two ion-electron equations representing each half-step either reduction or oxidation.
Eg O -2 means that it is an atom of oxygen that has an oxidation state of -2. The ion-electron equations must be. In order to be able to recognize redox reactions we need a method for keeping a careful account of all the electrons.
The answer key shows the correct answer as D but Im confused as to why.

Total 2 Average 5 5 Redox Reactions By Transfer Of Electrons At A Distance In All Redox Reactions Electrons Are Tra Redox Reactions Electrons Reactions

An In Depth Introduction To Balancing Redox Equations Complete With A Step By Step Guide To Make Sure Nothing Is Misse Redox Reactions Mcat Mcat Study Schedule

Limiting Reactant Lab Experiment By Threefourthsme Tpt Chemical Equation Equations Experiments

Question Video Identifying A Spectator Ion In A Simple Redox Reaction Nagwa
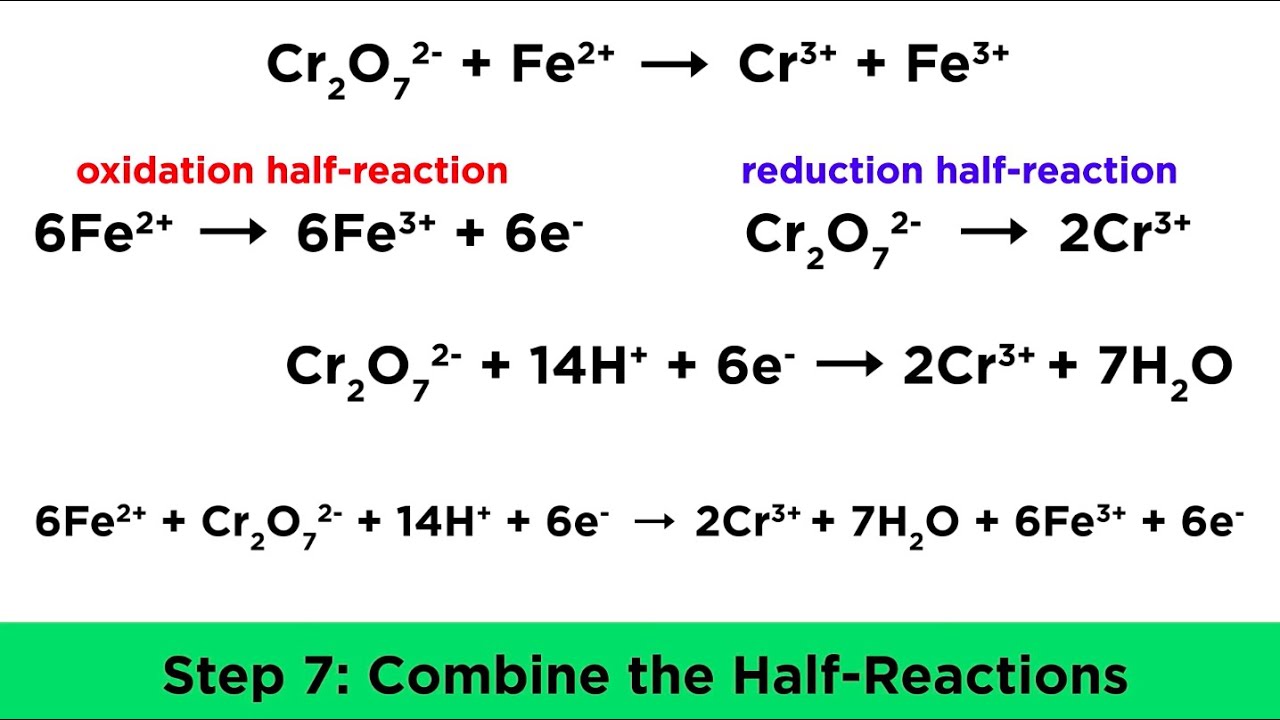
Balancing Redox Reactions In Acidic And Basic Conditions Youtube

Balancing Redox Equations In Alkaline Solution A Level Chemistry Tutor Youtube Equations Chemistry Tutor

Introduction To Balancing Redox Reactions On The Mcat Youtube

Oxidation Reduction Redox Reaction 1 Redox Reactions Reactions Oxidation

Redox Half Equations A Level Chemistry Tutor Aqa Edexcel Ocr Cie Chemistry Youtube Equations Tutor Chemistry

Identifying Redox Reactions Reason Prep

Oxidation Reduction Redox Reactions Balancing Redox Reactions Chemistry Net Chemistry Lessons Chemistry Classroom Chemistry Help

Students Will Be Practicing Identifying The Oxidizing Agent And Reducing Agent In A Redox Reaction By Completing Practices Worksheets Worksheets Reducing Agent

Ion Electron Method For Balancing Redox Reaction Redox Reactions Method Reactions

How To Tell If Redox Reduction Oxidation Reaction Is Spontaneous Examples And Practice Problems Youtube
Oxidation Reduction Reactions Redox

Ncert Solutions For Class 11 Chemistry Chapter 8 Redox Reactions 053 11th Chemistry Redox Reactions Chemistry
Post a Comment for "How To Identify A Redox Equation"